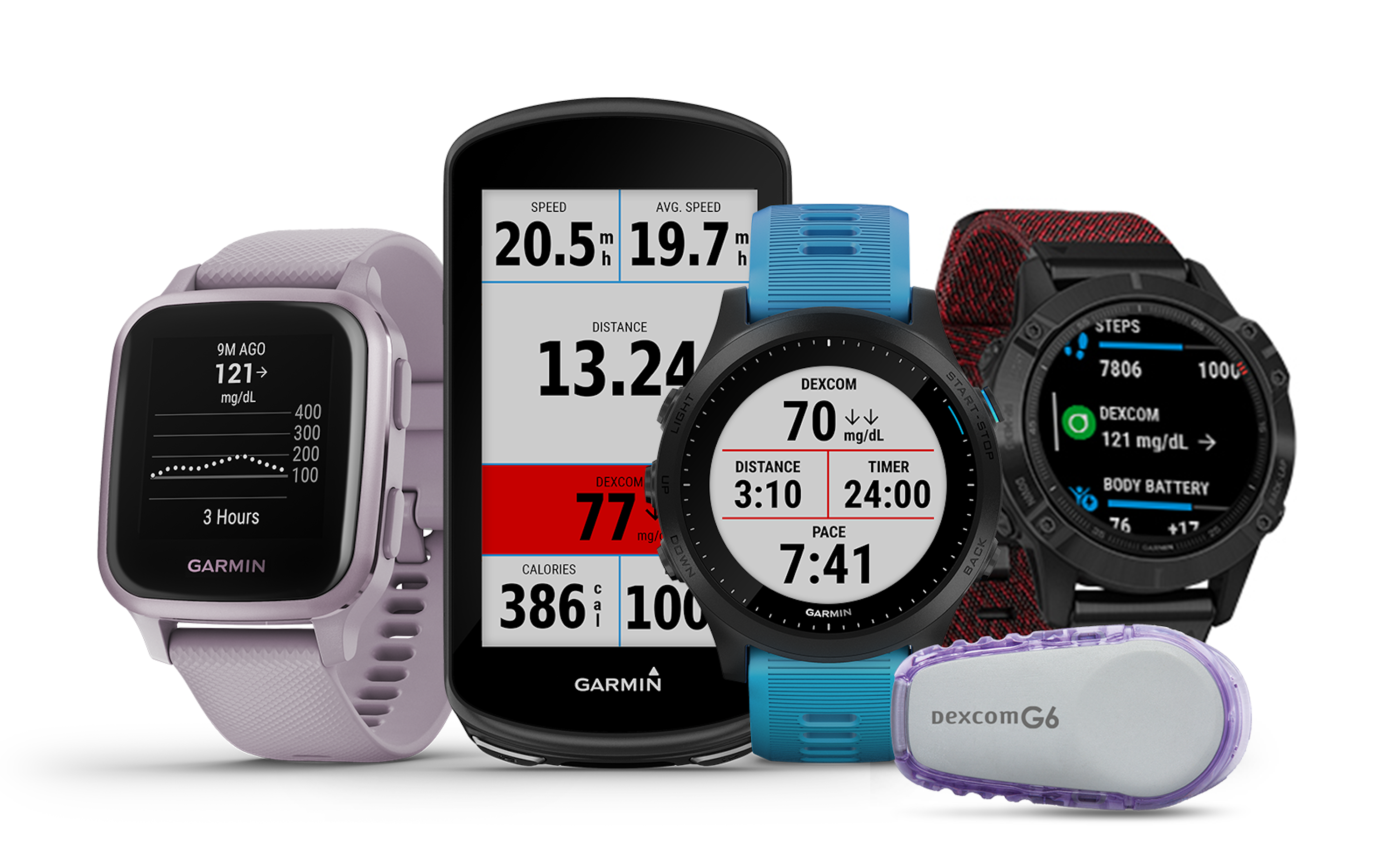
Dexcom taps Garmin for first real-time API integration
In July, Dexcom got FDA clearance to allow third parties to access its CGM data. To start, it is integrating with Garmin’s wearable devices.

In July, Dexcom got FDA clearance to allow third parties to access its CGM data. To start, it is integrating with Garmin’s wearable devices.

The company recently published new results on its MOBILE study, which found that CGM use increased time in range for people with Type 2 diabetes. New data shows people who stopped using a CGM saw time in range decrease.

The TSX Venture Exchange has a strong history of helping early-stage health and life sciences companies raise patient capital for research and development.

In a wide-ranging interview, Dexcom’s CEO shared more about the company’s push to prove its continuous glucose monitor can be used both for patients with Type 1 and Type 2 diabetes, and how the device-maker is handling a growing number of competitors.

Dexcom launched its first venture fund. The company, which makes continuous glucose monitors, plans to invest in glucose sensing technologies and metabolic monitoring.
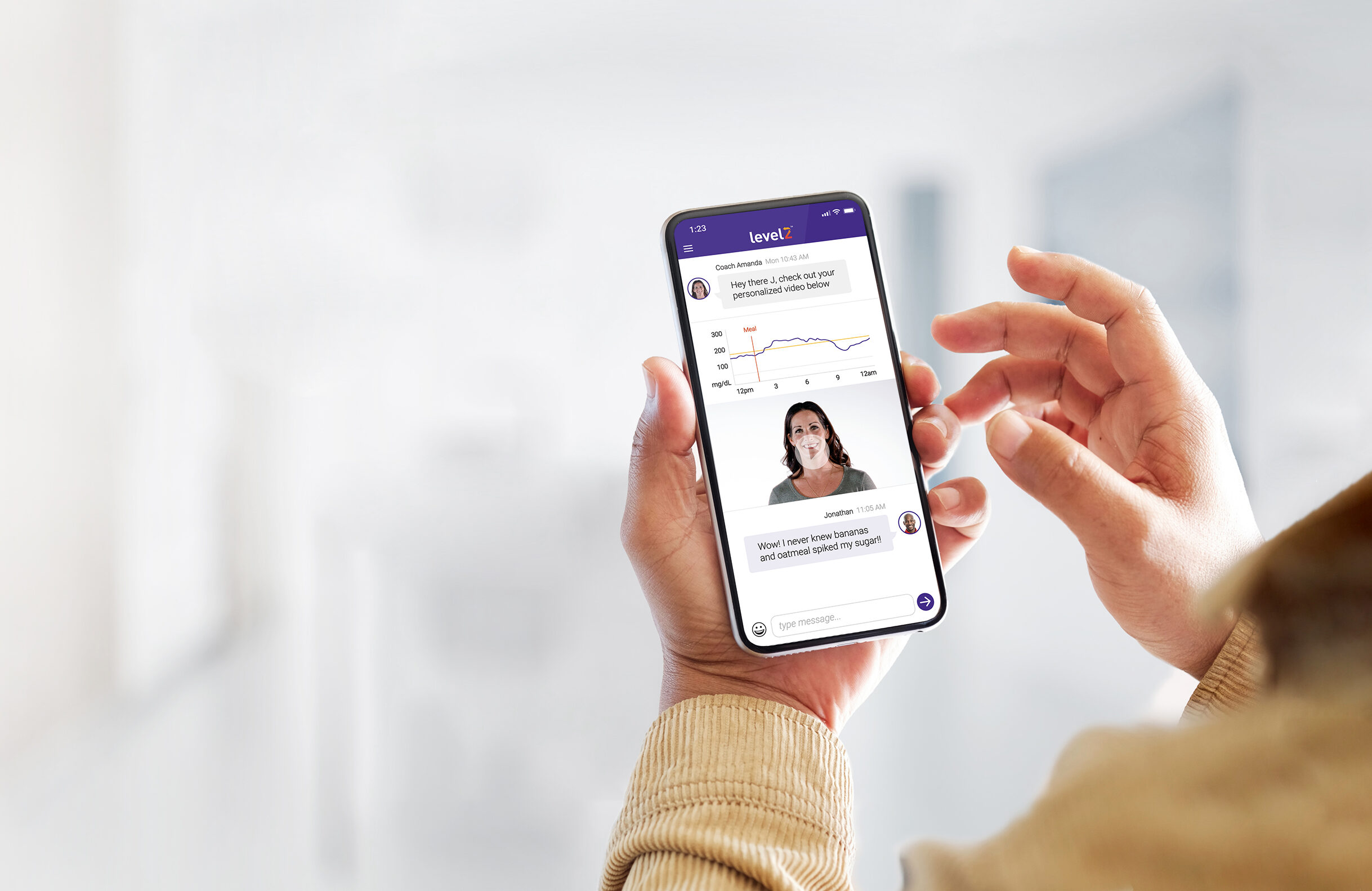
The company is rolling out Level2, a digital health program for patients with type 2 diabetes. It uses wearable devices and coaches to help users manage their health.

Abbott Laboratories received FDA clearance for its newest continuous glucose monitor, the FreeStyle Libre 2. The product is one of many in a growing market for CGMs.

In an era of escalating healthcare costs and a growing preference for natural, holistic approaches to health, The Impact Brands emerges as a collective of diverse brands dedicated to supporting overall wellness through natural means.

In an interview at the J.P. Morgan Healthcare Conference in San Francisco earlier this week, the CEO of Dexcom relayed a strong desire to sell CGM systems directly to the consumers with possible pilots this year.

Livongo and Dexcom struck a partnership on Monday that would combine Dexcom’s continuous glucose monitors with Livongo’s software. At the StartUp Health Conference, Livongo President Jennifer Schneider said its work with Dexcom would create a new stream of data for both companies.

The money will help the company launch its first insulin-management product by the end of this year, which is likely to face stiff competition from larger rivals.

Medical device maker Dexcom is partnering with Livongo such that users of Livongo that have diabetes and use Dexcom's continuous glucose monitoring system can sync their data on the Livongo platform.

At the Payer Insights sessions on Day 1 of ViVE 2024, a panel on prior authorization offered compelling insights from speakers who shared the positive developments in this area after years of mounting frustration. Speakers also shared challenges as they work with providers to figure out how policy developments and technology will work in practice.
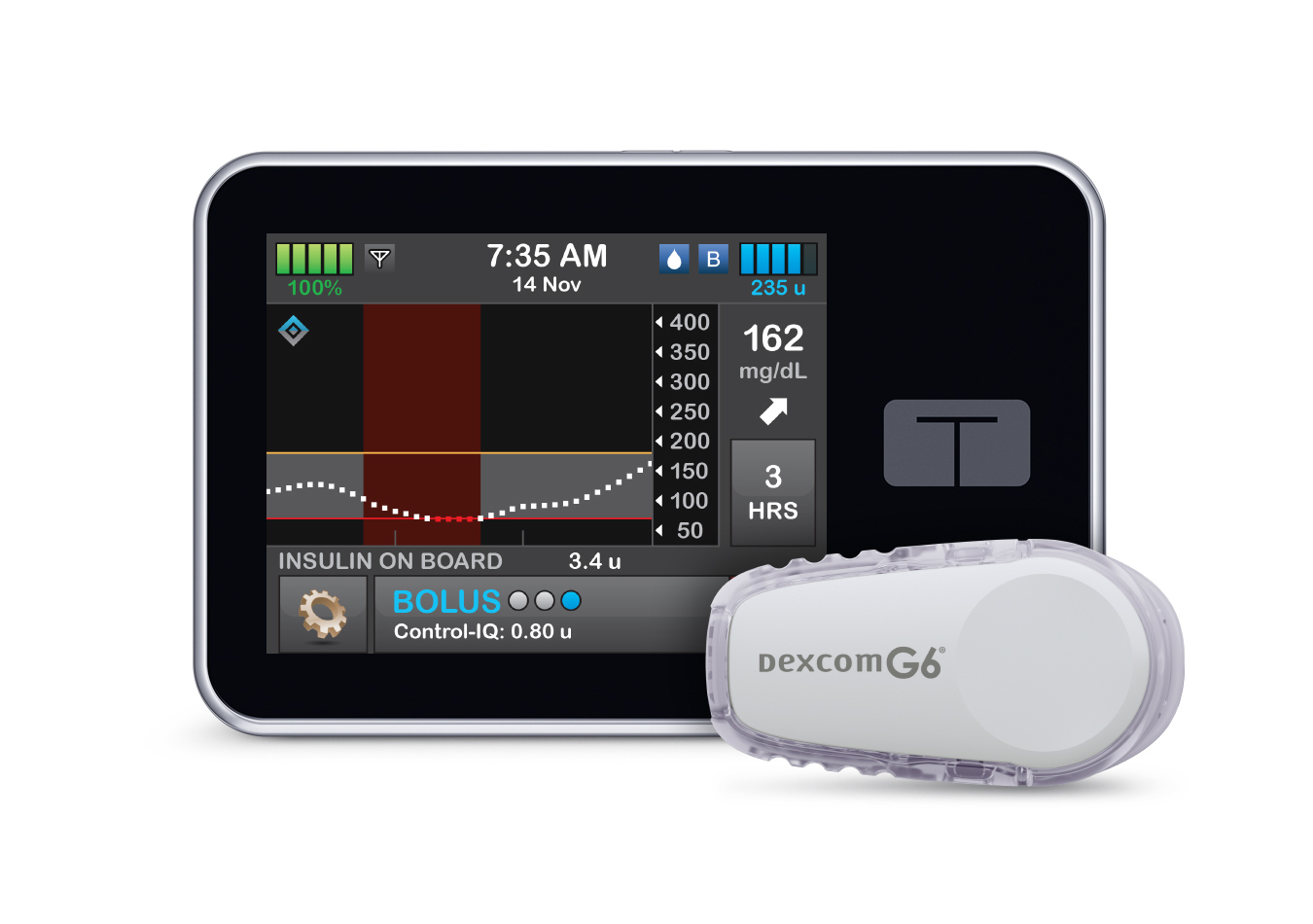
Tandem Diabetes’ technology will create a new approval pathway for predictive dosing software that can be used with other devices.

Scores of users took to Facebook to express their dismay, frustration and anger at Dexcom when the CGM device company failed to properly keep them updated after an IT outage prevented type 1 diabetes patients from sharing data.

Find Care, which was launched one year ago, was initially started to help patients find and identify location specific healthcare services through the Walgreens app and website.

On the day that an FDA official exhorts industry to develop interoperable diabetes devices, Medtronic announces that it is teaming up with a nonprofit group to do just that.

Fitabase CEO Aaron Coleman said he sees the integration being particularly useful to researchers looking at how behavioral recommendations in areas like physical activity and sleep can affect glucose levels to create more tailored care plans for diabetic patients.