System1 Biosciences raises $25M in Series A round for neurotherapeutics discovery platform
System1 Biosciences' platform combines stem cell lines from patients with brain diseases with a three-dimensional in vitro system and data analytics.

System1 Biosciences' platform combines stem cell lines from patients with brain diseases with a three-dimensional in vitro system and data analytics.
The FDA approved the cannabis-based drug for treating seizures in two severe forms of epilepsy.

Canada has a proud history of achievement in the areas of science and technology, and the field of biomanufacturing and life sciences is no exception.
Bloom Science plans to have its proprietary gut bacteria in the clinic for hard-to-treat patients in the next 12-18 months.

By passively collecting seizure data, Empatica's device could help patients share medical information with clinicians and make that data easier to manage.

Medical researchers frequently spend the majority of their time formatting research data and 10 percent of their time actually using it. Blackfynn hopes its platform will aid translational research and therapeutic product development.

NeuroVice's device is designed to provide a safe way to protect tongues and minimize salivation during seizures.

In an era of escalating healthcare costs and a growing preference for natural, holistic approaches to health, The Impact Brands emerges as a collective of diverse brands dedicated to supporting overall wellness through natural means.
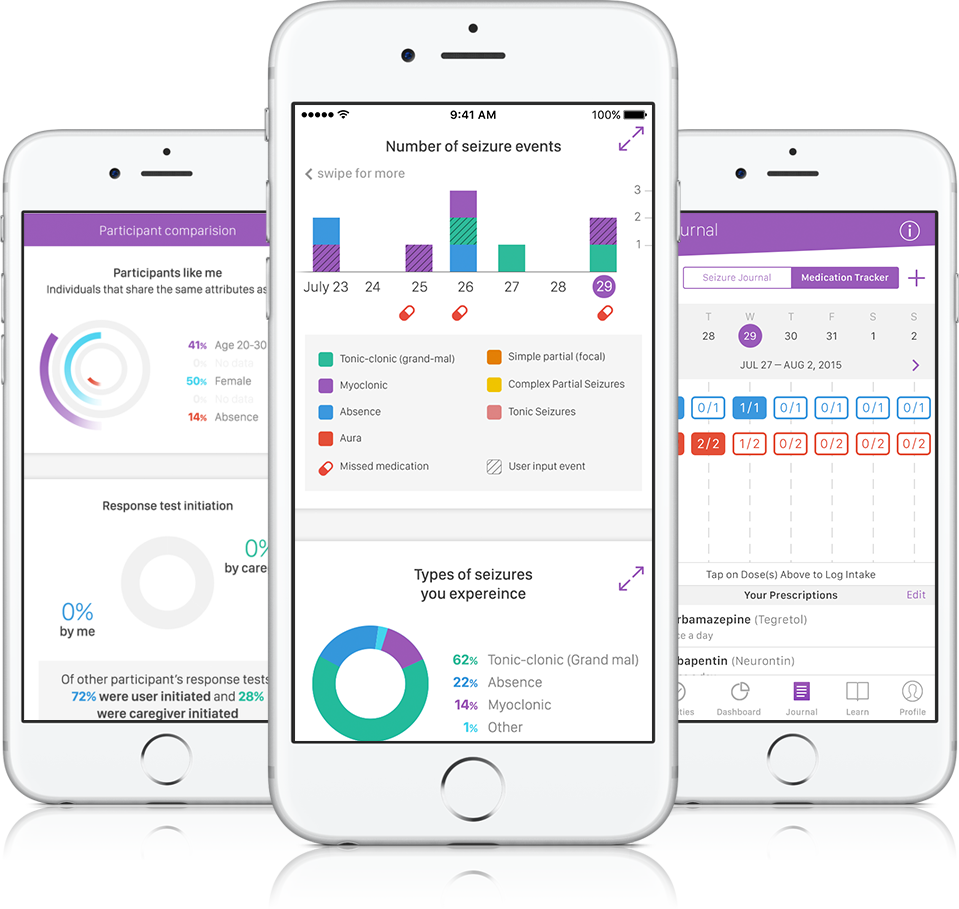
The goal is to track the severity and length of the seizure using sensors such as an accelerometer and a gyroscope as well as a memory test when the user comes out of the event.
Aprecia Pharmaceuticals is getting closer to commercializing SPRITAM, its 3D printed formulation of levetiracetam, which is a well-established medication used by patients with epilepsy.

The day after news broke that Turing Pharmaceuticals' Daraprim has a new competitor on the market, CEO Martin Shkreli announced the company's gotten fast-track status for its experimental epilepsy drug.

Turing isn't just buying drugs and bumping up prices. It is actually putting a focus on new drugs, which is exemplified with its recent investigational new drug application.

This eBook, in collaboration with Care Logistics, details how hospitals and health systems can facilitate more effective decision-making by operationalizing elevated awareness.

The Los Angeles-based company developing its drug-resistant epilepsy system has officially backed out of its IPO plans.

New, wearable technology not only helps monitor symptoms automatically, it may be able to predict seizures and disease progression in patients with neurological disorders.

The FDA just approved the first 3D printed drug. Are there other applications for this technology?

The FDA has approved a 3D-printed drug designed to treat epileptics, OraSure Technologies' Ebola test gets emergency use authorization.

Here’s a story with a slight Cyborg ring to it: An implantable brain device that can potentially restore the memory of patients with traumatic brain injury, Alzheimer’s disease, epilepsy and related conditions is being developed at the government’s Lawrence Livermore National Laboratory. The technology sits at the nexus of human, neuron-based memory and digital, computer chip-based memory: The device […]