Contineum Therapeutics, a biotech company developing drugs for applications in neurological disorders as well as immunology and inflammation indications, has raised $110 million in IPO cash to support a pipeline that includes a lead program in early clinical development for fibrosis.
San Diego-based Continuem needed to lower its expectations in order to proceed with the stock sale. In preliminary financial terms set earlier this week, the company aimed to offer 8.8 million shares for $16 to $18 each, which would have raised $149.6 million at the pricing midpoint. But late Thursday, Contineum priced its offering of 6.75 million shares at $16 apiece. Those shares will trade on the Nasdaq under the stock symbol “CTNM.”
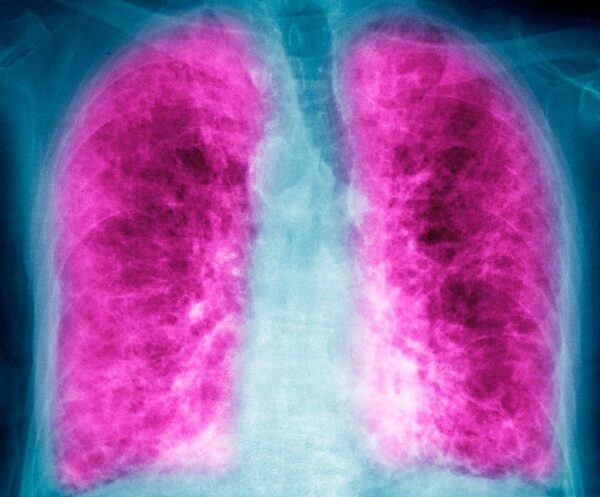
Contineum’s lead wholly owned asset, PIPE-791, is in Phase 1 testing for idiopathic pulmonary fibrosis (IPF), a chronic disorder in which the buildup of scar tissue, or fibrosis, leads to a progressive loss of lung function that becomes fatal. The approved IPF drugs only slow the disease’s progression and they bring a wide range of systemic side effects. The need for new and better treatments has attracted many companies into IPF research.

At ViVE 2024, Panelists Share Prior Authorization Progress and Frustration in Payer Insights Program
At the Payer Insights sessions on Day 1 of ViVE 2024, a panel on prior authorization offered compelling insights from speakers who shared the positive developments in this area after years of mounting frustration. Speakers also shared challenges as they work with providers to figure out how policy developments and technology will work in practice.
The target of PIPE-791 is lysophosphatidic acid 1 (LPA1), a receptor that contributes to fibrosis when activated. Contineum’s drug is a small molecule designed to block this receptor. Other companies developing LPA1 receptor blocking drugs include Bristol Myers Squibb, AbbVie, Horizon Therapeutics, and Structure Therapeutics. Other companies are pursuing other targets for IPF.
Multiple sclerosis is another disease target for PIPE-791. In the IPO filing, Contineum notes that levels of LPA, a pro-inflammatory protein, are elevated in MS patients, and may contribute to the progression of the disease. The Contineum drug’s ability to penetrate into the brain offers the potential to treat MS. In preclinical research, Contineum said blocking the LPA1 receptor reduced neuroinflammation and promoted the formation of new myelin sheaths on axons that lost this protective layer.
MS is also the focus of PIPE-307, a Contineum drug candidate licensed by Johnson & Johnson. This small molecule is designed to selectively block the M1 receptor, which has potential applications in MS and depression. Though J&J now has global rights to develop PIPE-307 for all indications, the agreement permits Contineum to develop the molecule for relapsing and remitting forms of MS. A Contineum Phase 2 study is ongoing.
Contineum, which traces its origins to a startup creation engine of Versant Ventures, was formerly known as Pipeline Therapeutics. At the end of last year, the company changed its name. Contineum reported a $15.5 million cash position along with $109.6 million in marketable securities at the end of 2023. According to the IPO filing, Contineum plans to spend about $66 million on lead program PIPE-791, taking the drug through Phase 2 testing in IPF and progressive MS.

The Impact Brands: Empowering Wellness Through Natural and Holistic Solutions
In an era of escalating healthcare costs and a growing preference for natural, holistic approaches to health, The Impact Brands emerges as a collective of diverse brands dedicated to supporting overall wellness through natural means.
Another $16.2 million is planned for the completion of the Phase 2 test of PIPE-307 in relapsing and remitting MS. Continuem did not specify timelines for those plans. In the filing, the company said it expects its capital is sufficient to last through the end of 2028. That projection assumes Contineum receives no additional payments from the J&J collaboration and the company does not exercise its option to fund a portion of Phase 3 development of the partnered drug in any indication.
Image: Cavllini James/BSIP/Education Images/Universal Images Group, via Getty Images












