From Recalls to Reliability: Tools to Ensure Software Safety in MedTech
Cutting-edge technology empowers developers to proactively detect and mitigate issues before they cause problems, reducing recalls and fostering trust in the industry.

Cutting-edge technology empowers developers to proactively detect and mitigate issues before they cause problems, reducing recalls and fostering trust in the industry.

Three tips to succeed in a highly regulated environment

This eBook, in collaboration with Care Logistics, details how hospitals and health systems can facilitate more effective decision-making by operationalizing elevated awareness.

The MedTech industry is poised for breakthroughs, owing to the rapid integration of digital ecosystems and technologies like AI and cloud.

Each track will spotlight a different specialty area including: oncology, cardiology, women's health and preventing burnout/supporting operational efficiency.

Providers who don't take advantage of AI will get "left behind," said Dr. Shantanu Gaur, founder and CEO of Allurion Technologies, during a panel discussion at INVEST Digital Health.
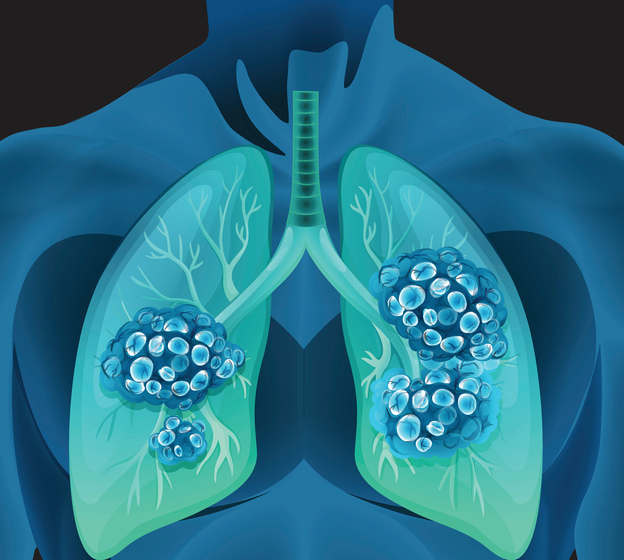
Johnson & Johnson released research this month showing that its system for robotic-assisted bronchoscopy helped physicians achieve an over 15% improvement in overall diagnostic yield compared to traditional bronchoscopy. This could mean that fewer patients will have to “wait and see” while their cancer potentially gets worse.

At the Payer Insights sessions on Day 1 of ViVE 2024, a panel on prior authorization offered compelling insights from speakers who shared the positive developments in this area after years of mounting frustration. Speakers also shared challenges as they work with providers to figure out how policy developments and technology will work in practice.

Investment trends will be a big theme at the HLTH event at the Las Vegas Convention Center October 8-11. In the runup to the conference, investors from Bessemer Venture Partners, Cleveland Clinic Innovations, Third Culture Capital and Intuitive Ventures offered their takes on 2023 investments.

Medtech companies should be particularly mindful of the following legal and regulatory compliance issues. This is especially true for companies transitioning to data-based services.

From streamlining long-held manual processes to creating entirely new diagnostic devices, new tech is cropping up all over the medtech industry—resulting in a real-world impact on patients' quality of life.

Canada has a proud history of achievement in the areas of science and technology, and the field of biomanufacturing and life sciences is no exception.

Executives from Huma, Abbott, Smith & Nephew and AliveCor share how they are using real world evidence collection to support remote patient monitoring, maximize patient engagement and navigate the regulatory landscape.

Huma plays a vital role in leveraging its digital infrastructure to help medical device companies navigate the regulatory complexities of generating RWE.

The industry faces challenges from inflation to labor shortages, according to an annual report from EY. However, there are grounds for optimism and areas to focus on, such as patching up supply chains.

A look at how tapping real world evidence from medical devices and patients could be a game changer, improving patient-clinician communication, supporting earlier interventions and helping patients manage their condition.

An upcoming webinar sponsored by Huma will highlight three aspects of remote monitoring: real world data collection, supercharging patient engagement and navigating the regulatory landscape. Register today!