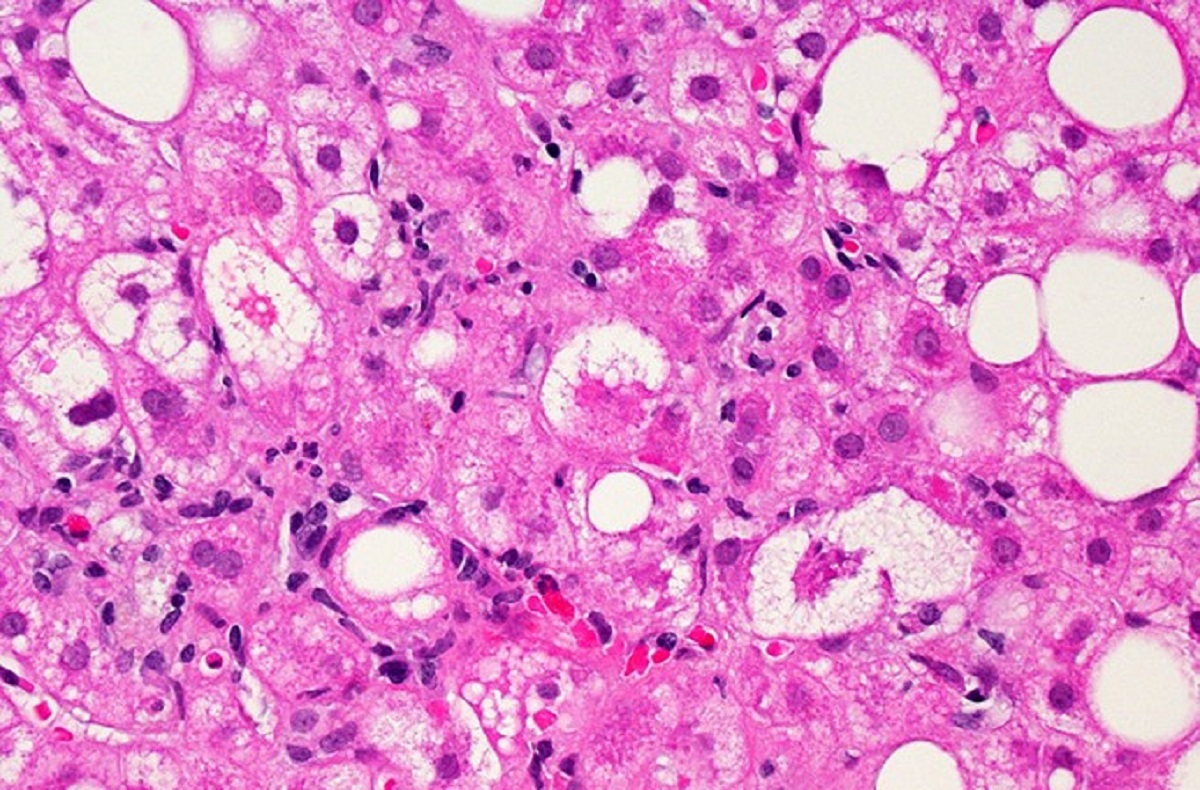
The history of drug research in the liver disease non-alcoholic steatohepatitis (NASH) is littered with clinical trial failures, but Akero Therapeutics is now trumpeting a Phase 2 win. Crucially, the clinical trial success was determined using new methodology the FDA is requiring NASH drug developers meet before seeking drug approval.

When Investment Rhymes with Canada
Canada has a proud history of achievement in the areas of science and technology, and the field of biomanufacturing and life sciences is no exception.
According to preliminary results released Tuesday, the Akero drug efruxifermin led to at least one stage of improvement in a measure of fibrosis, or liver scarring, that develops from the fatty liver disease. That result, along with no worsening of the disease by week 24 of the study, was achieved by 41% of those in the high dose group and 39% of those given the low dose. By comparison 20% of those in the placebo group achieved the study goal.
South San Francisco-based Akero also reported success against a secondary goal of achieving NASH resolution without worsening of fibrosis. At the high dose, 76% of patients achieved that mark; 47% of those given the low dose met that goal. Just 5% of those in the placebo arm met this secondary endpoint. Shares of Akero opened Tuesday at $28.04 apiece, up more than 128% from Monday’s closing price.
NASH is an advanced form of liver disease characterized by buildup of fat in the organ that leads to inflammation and fibrosis. Risk factors associated with the development of NASH include obesity and high cholesterol. There are no FDA-approved drugs for NASH, which is mainly treated with dietary adjustments and other lifestyle changes. At the most advanced stage of the disease, the only treatment is a liver transplant.
Efruxifermin is a fusion protein engineered to serve as an analog of fibroblast growth factor 21 (FGF21), a hormone that protects against cellular stress and regulates the metabolism of fats, carbohydrates, and proteins. Akero licensed the drug from Amgen in 2018. The preliminary data released Tuesday are from a Phase 2b test enrolling 128 adults whose biopsy-confirmed NASH has reached fibrosis stage 2 or 3 (there are four stages, with stage 4—cirrhosis—being the most severe). Those participants were randomly assigned to receive either a high or low dose of the once-weekly injectable Akero drug, or a placebo, for 24 weeks.

Unlocking Transparency in PBM Pricing
The TSX Venture Exchange has a strong history of helping early-stage health and life sciences companies raise patient capital for research and development.
Efficacy of Akero’s drug was assessed by liver biopsies that were independently scored by two pathologists, with a third pathologist available to weigh in if the first two did not reach consensus. The FDA is now requiring this consensus liver biopsy analysis for NASH drugs. When the FDA rejected Intercept Pharmaceuticals’ NASH contender, obeticholic acid, in 2020, the agency asked for data analysis done according to this “consensus read methodology.” Intercept reported results from such an analysis in July, which the company said should support resubmission of its new drug application. The Phase 3 failure of Genfit’s NASH drug led the Nord, France-based company to abandon pursuit of a treatment for that disease in favor of a rarer liver disorder. Last year, Genfit sold the rights to that drug, elafibranor, to Ipsen.
To succeed in Phase 3, the FDA requires a NASH drug to achieve one of two goals: fibrosis improvement of one stage or more with no worsening of the disease symptoms, or NASH resolution with no worsening of fibrosis. The European Medicines Agency requires that both of those goals be met to support marketing authorization of a NASH drug.
While the preliminary results for Akero’s NASH drug show better results for the high dose compared to the low dose, that advantage does not hold up when measuring for fibrosis improvement of two stages or more. The biotech reported in an investor presentation that 16% of patients who received the low dose showed such improvement, a benchmark achieved by 15% of patients in the high dose group. By comparison, 5% of patients in the placebo arm showed fibrosis improvement of two stages or more.
The Akero drug was well tolerated by patients. The most frequent adverse events reported from the study included diarrhea, nausea, increased appetite, and frequent bowel movements. A drug-related serious adverse event of inflammation of the esophagus was reported in one patient in the high-dose group. Akero said this patient had a history of gastroesophageal reflux disease.
“We believe the data are very compelling and show [efruxifermin’s] potential to meet the critical, global unmet need for patients by intervening across stages of disease progression, potentially addressing both early-stage metabolic drivers and later-stage inflammation and fibrosis,” Andrew Cheng, president and CEO of Akero, said in a prepared statement.
A separate Phase 2b test of the Akero drug is ongoing in NASH patients who have reached compensated cirrhosis, Child-Pugh class A, which is the least severe classification of the most advanced stage of NASH. Akero said it expects to report results from this mid-stage study in the second half of next year. This study has also expanded to include the treatment of patients with the Akero drug along with a type of diabetes drug called a GLP-1 agonist. Results for this group are expected in the first half of 2023.
Public domain image by the Flickr user NIH Image Gallery












