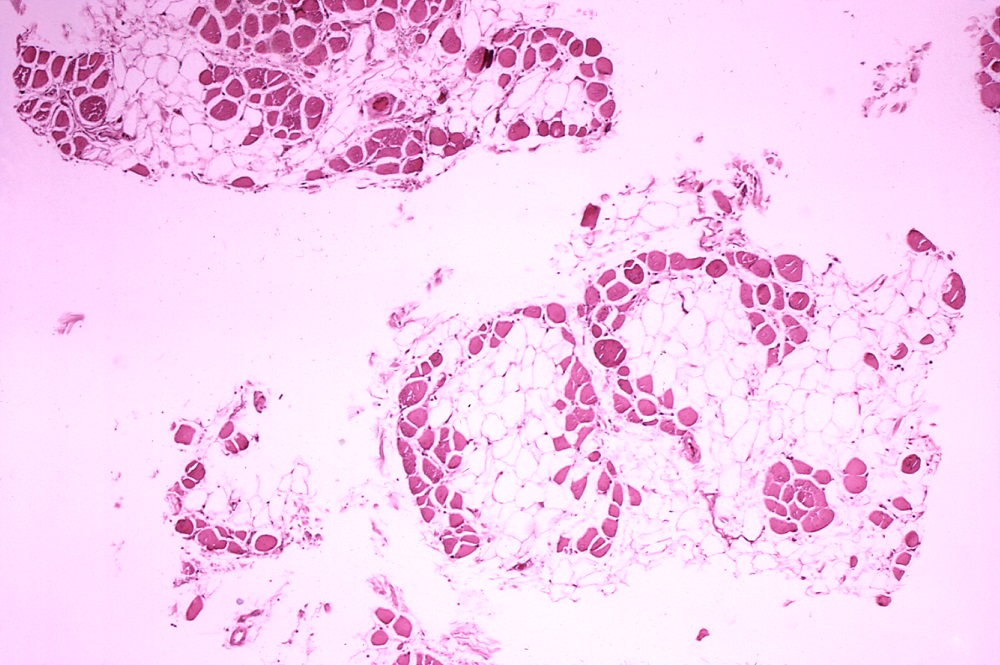
The rare disorder Duchenne muscular dystrophy is treated with genetic medicines that get skeletal muscle cells to produce a version of the key protein that they lack. Though these therapies slow progression of this disease that robs patients of the ability to walk, patients typically die from Duchenne’s effects on the heart and breathing. Biotech company PepGen is developing a drug that delivers its therapeutic payload not only to skeletal muscle, but also to the diaphragm, cardiac muscle, and the central nervous system. PepGen’s scientists believe this broader delivery capability gives it an edge and the clinical-stage company has joined the public markets as it sets out to prove its approach.

At ViVE 2024, Panelists Share Prior Authorization Progress and Frustration in Payer Insights Program
At the Payer Insights sessions on Day 1 of ViVE 2024, a panel on prior authorization offered compelling insights from speakers who shared the positive developments in this area after years of mounting frustration. Speakers also shared challenges as they work with providers to figure out how policy developments and technology will work in practice.
Cambridge, Massachusetts-based PepGen initially planned to offer 7.2 million shares in the range of $13 to $15 each, which would have raised $108 million at the $14 per share midpoint. The company was able to raise that amount, but it had to offer more shares do so. Late Thursday, PepGen priced its offering of 9 million shares at $12 apiece. Those shares began trading on the Nasdaq Friday under the stock symbol “PEPG.”
For diseases with a known genetic cause, one therapeutic approach is an antisense oligonucleotide, a type of therapy made from small pieces of synthetic DNA or RNA. The four FDA-approved therapies for Duchenne—three from Sarepta Therapeutics and one from NS Pharma—are all antisense oligonucleotides. PepGen said in its IPO filing that the limiting factor for oligonucleotide-based drugs is delivery.
“On their own, oligonucleotides therapeutics are not readily distributed to heart and skeletal muscle, the key tissues affected in neuromuscular diseases, and are not efficiently taken up into these cells,” the company said. “To address this challenge, we engineered our proprietary EDO technology to optimize tissue penetration, cellular uptake and nuclear delivery, which we believe may enhance the therapeutic activity of oligonucleotides and improve the tolerability of these genetic medicines.”
EDO stands for Enhanced Delivery Oligonucleotide. This technology platform engineers peptides so that they can better carry oligonucleotide cargos to desired tissue destinations. In preclinical research, PepGen has shown its technology can transport oligonucleotides into tissue types that include smooth, skeletal, and cardiac muscle, as well as the central nervous system.

The Impact Brands: Empowering Wellness Through Natural and Holistic Solutions
In an era of escalating healthcare costs and a growing preference for natural, holistic approaches to health, The Impact Brands emerges as a collective of diverse brands dedicated to supporting overall wellness through natural means.
PepGen’s lead program, called PGN-EDO51, is intended to treat Duchenne patients whose disease can be addressed by getting the cell’s protein-making machinery to skip exon 51, one of the segments of a gene containing the code that is translated into a protein. This approach leads to a shortened version of the key muscle protein that Duchenne patients lack.
Exon skipping is the same approach taken by Sarepta’s first FDA-approved Duchenne therapy, Exondys 51. Sarepta is developing a next-generation exon 51-skipping drug that uses cell-penetrating peptides to improve delivery. That therapeutic candidate, SRP-5051, is currently in Phase 2 testing. PepGen said in its IPO filing that it conducted a monkey study comparing its experimental drug against a molecule that’s equivalent to Sarepta’s next-generation exon-skipper. According to PepGen, results showed a greater than 70% increase in exon 51 skipping in skeletal muscle, including the diaphragm. The PepGen therapy also showed greater activity.
Last month, PepGen began a Phase 1 single-ascending dose study enrolling healthy volunteers in Canada. Preliminary data from the clinical trial are expected by the end of this year. The EDO platform has yielded a second program, PGN-EDODM1, which the biotech is developing as a treatment for myotonic dystrophy type 1 (DM1), a rare muscle disorder that currently has no FDA-approved treatments. PepGen said in its prospectus that it expects to submit an investigational new drug application for a DM1 therapy in the first half of next year.
A second Duchenne therapy, PGN-ENDO53, is being developed to address patients who can be treated by skipping exon 53. PepGen said it expects to begin a test of that drug in monkeys in the second half of 2022. Besides further Duchenne research, PepGen said it plans to study potential applications of its technology in additional indications that include neuromuscular diseases and neurological disorders.
The science behind PepGen’s drugs is based on more than a decade of research from the labs of Matthew Wood, a professor of neuroscience at the University of Oxford, and Mike Gait, emeritus scientist at the Medical Research Council of United Kingdom Research and Innovation. PepGen was founded in 2018; in 2020, the biotech closed a $45 million Series A round of funding. Prior to the IPO, PepGen had raised $163.9 million, the company said in its prospectus. Its most recent financing was a $112.5 million crossover round that closed last August.
RA Capital is PepGen’s largest shareholder with a 23.7% stake after the IPO, according to the prospectus. Oxford Sciences Enterprises, a firm that takes a stake in Oxford University science spinouts, owns 18.3% of the company. As of the end of 2021, PepGen had $132.8 million in cash, the prospectus shows. Combined with the IPO proceeds, PepGen plans to spend about $70 million on completing Phase 1 testing of PGN-EDO51 and continuing on to a planned Phase 2a study. Another $45 million is set aside for completing a Phase 1/2 clinical trial for PGN-EDODM1, while about $30 million is earmarked for further development of its drug pipeline and platform. Following the IPO, PepGen estimates its cash holdings will carry the company into the first half of 2025.
Bausch + Lomb raises $630M in return to the public markets
Bausch + Lomb is trading on the New York Stock Exchange again after completing an IPO that comes 15 after the iconic eye products company was taken private. On Thursday evening, the company priced its offering of 35 million shares at $18 apiece, well below the $21 to $24 price range the company had previously set. In addition to its debut on the NYSE, Vaughan, Ontario-based Bausch + Lomb also listed shares on the Toronto Stock Exchange. The stock trades on both exchanges under the stock symbol “BLCO.”
The Bausch + Lomb IPO marks the company’s separation from Bausch Health Companies (formerly Valeant Pharmaceuticals), the company that acquired it in 2013. Bausch + Lomb was initially taken private in 2007 with its acquisition by private equity firms for $4.7 billion. Bausch + Lomb won’t receive any of the IPO proceeds, which will go to Bausch Health. In advance of the IPO filing in January, Bausch Health CEO Joe Papa said that his firm will use the cash from the IPO to pay down debt.
Public domain image by the Centers for Disease Control and Prevention












