Eli Lilly’s cardiometabolic drug portfolio is expanding with newly approved therapies. To meet demand for those therapies and additional products to come, the company is building a new $2.5 billion manufacturing site in Germany.
Construction on the new plant is on track to begin next year. Lilly expects the site will start operations in 2027, becoming its sixth manufacturing site in Europe. The company said it has invested more than $11 billion in its global manufacturing capabilities in the past three years to support the production of medicines across its portfolio.

When Investment Rhymes with Canada
Canada has a proud history of achievement in the areas of science and technology, and the field of biomanufacturing and life sciences is no exception.
Some of Lilly’s capital investments have been closer to the Indianapolis-based drugmaker’s home. Last year, the company committed more than $2 billion to two new facilities in Lebanon, Indiana for the manufacturing of existing products and future ones, the company said in its annual report. Lilly also invested more than $1 billion in a new facility in Concord, North Carolina, for the manufacturing of injectable products and devices. Earlier this year, Lilly pledged to spend an additional $450 million to expand capacity at a site in Research Triangle Park that also makes injectable products, including new blockbuster medicine Mounjaro.
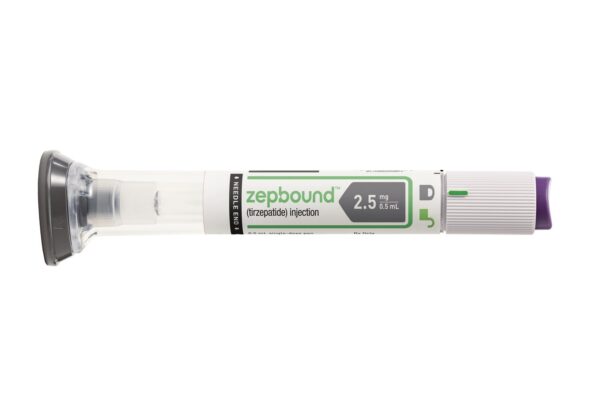
Mounjaro won its initial FDA approval last year for treating patients with type 2 diabetes, quickly becoming a blockbuster seller in that indication. Earlier this month, the FDA expanded the drug’s approval to include chronic weight management, where the once-weekly injectable will be marketed under the brand name Zepbound.
By expanding its manufacturing capacity, Lilly could avoid some of the manufacturing constraints that encountered by rival Novo Nordisk and its weight loss drug, Wegovy. Demand has outpaced the Danish drugmaker’s ability to supply the drug, leading the company to turn to contract manufacturers to increase its production capacity. Earlier this month, Novo Nordisk announced plans to invest more than 42 billion Danish kroner (about $6.1 billion) to expand an existing manufacturing facility in Denmark for current and future demand for products that include its GLP-1 drugs, Wegovy and Ozempic for type 2 diabetes.
In addition to Lilly’s plans to build manufacturing capacity in Germany, the company also said it will invest up to $100 million to bolster its presence in the country’s early-stage biotech ecosystem. These investments will include expanding Lilly’s engagement with incubators and accelerators. Lilly said it will also strengthen ties to academics and other innovators.

Using Informed Awareness to Transform Care Coordination and Improve the Clinical and Patient Experience
This eBook, in collaboration with Care Logistics, details how hospitals and health systems can facilitate more effective decision-making by operationalizing elevated awareness.
“As we look to usher in the next generation of medicines, we’re looking for people and partners who share our passion for improving health outcomes,” Ilya Yuffa, executive vice president and president, Lilly International, said in a prepared statement. “With this new investment, we hope to spark a new era of collaboration and innovation in Germany and the European Union defined by our shared purpose to find lasting solutions for patients.”
Photo by Eli Lilly












