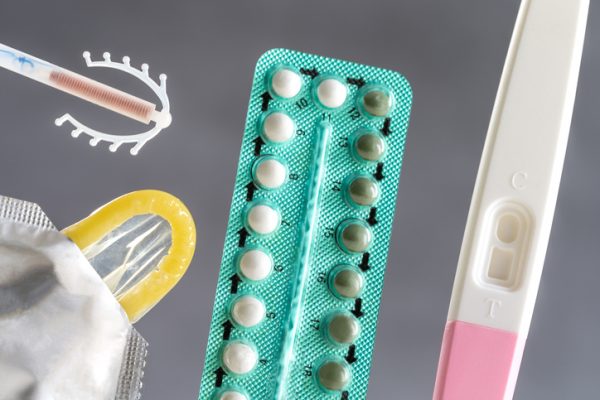
More than 30 birth control products have cost-sharing requirements or coverage exclusions, a violation of the Affordable Care Act, according to an investigation by the House Committee on Oversight and Reform into health plans and pharmacy benefit managers (PBMs).
The findings, revealed this week in a staff report, show that the insurers and PBMs are not abiding by the Affordable Care Act (ACA), which requires issuers and health plans to cover U.S. Food and Drug Administration (FDA)-approved contraceptive products without requiring patients to pay out-of-pocket, which is also referred to as cost-sharing.

When Investment Rhymes with Canada
Canada has a proud history of achievement in the areas of science and technology, and the field of biomanufacturing and life sciences is no exception.
The investigation also found that companies denied exceptions requests on average four or more times out of ten, raising barriers to accessing birth control, according to a news release issued Tuesday.
“In the wake of the extreme Supreme Court decision to overturn Roe v. Wade, the ability to decide if and when to become pregnant has never been more important,” said Rep. Carolyn B. Maloney, the Chairwoman of the Committee on Oversight and Reform in a news release.
The investigation is the first congressional report of contraceptive coverage in the private health insurance market. In May, the Committee requested information from five insurers: UnitedHealth, Anthem, Aetna, Cigna and Humana and four pharmacy benefit managers: CVS Caremark, Express Scripts, OptumRx and Prime Therapeutics as part of the investigation.
“The Biden-Harris Administration recently issued important guidance to enhance contraceptive coverage under the ACA, and I urge the Administration to further update their guidance to address the concerns identified in this report,” Maloney said.

Using Informed Awareness to Transform Care Coordination and Improve the Clinical and Patient Experience
This eBook, in collaboration with Care Logistics, details how hospitals and health systems can facilitate more effective decision-making by operationalizing elevated awareness.
The report found cost-prohibitive practices for several products. For example, the companies reported cost-sharing obligations of up to $178 per month for certain non-pill contraceptives like the Twirla patch, and approximately $218 per month for certain birth control pills.
The cost-sharing practices disproportionately affect lower income patients, according to the report. Four of the 17 products are non-pill products, which are disproportionately used by patients with less income and non-white patients.
“Unfortunately, accessibility is often determined by an individual’s particular health care coverage, which (in the case of private insurance) is often dependent on the individual’s job,” said Bethany Corbin, senior counsel at Nixon Gwilt Law, who advises femtech and healthcare innovation companies and hosts the Legally Femtech podcast.
Corbin said the report shows how “money – not medical necessity – can become the driving factor,” instead of medical advice and patient needs. “I’m grateful for the light that is finally being shown on the disproportionate impact health plans and PBMs have on women’s reproductive choices,” Corbin said.
When a patient tries to submit a request for an exception so that they can pay less out of pocket, they are met with significant delays, up to 15 days, the report found. One company reported a delay of up to 15 days to process exception requests, and two companies requested that providers document prior medications a patient has unsuccessfully tried in order for that patient to receive an exception.
The staff report recommends that the Department of Health and Human Services, the Department of Labor, and the Department of the Treasury issue guidance clarifying that all FDA-approved contraceptive products that do not have a therapeutic equivalent should be covered without cost-sharing as part of every plan or formulary.
Photo: Getty Images












