Astellas Pharma is hunting for drugs to make up for the decline in revenue facing its top-selling product as it loses patent protection in the next few years. It sees a strong contender in an Iveric Bio drug candidate for a common vision loss disorder, and the Japanese pharmaceutical company has agreed to acquire the biotech in a $5.9 billion deal that comes ahead of an expected FDA decision on the therapy.
Under terms of the deal announced Sunday evening, Tokyo-based Astellas will pay $40 cash for each share of Iveric. That price represents a 21.6% premium to the stock’s closing price on Friday. But the biotech’s share had already been on a steady climb since mid-February when FDA approval of a rival drug provided validation for Iveric’s approach. The agency that month also accepted Iveric’s new drug application, setting an August 19 target date for a decision on the biotech’s drug, avacincaptad pegol.

At ViVE 2024, Panelists Share Prior Authorization Progress and Frustration in Payer Insights Program
At the Payer Insights sessions on Day 1 of ViVE 2024, a panel on prior authorization offered compelling insights from speakers who shared the positive developments in this area after years of mounting frustration. Speakers also shared challenges as they work with providers to figure out how policy developments and technology will work in practice.
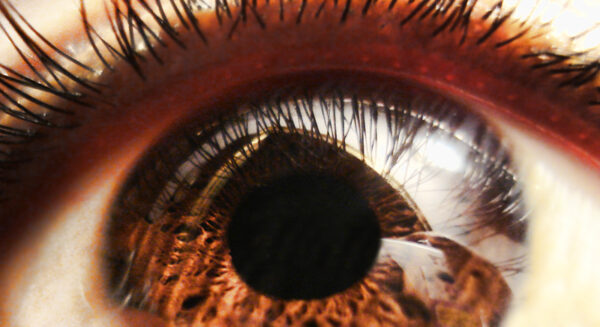
The Iveric drug, which has been given the brand name Zimura, is a potential treatment for geographic atrophy (GA), a condition that develops in patients with age-related macular degeneration. GA leads to lesions that destroy retinal cells, leading to a loss of central vision that progressively worsens to blindness. Parsippany, New Jersey-based Iveric estimates that 1.6 million people in the U.S. have this condition, which is associated with excessive activity of the complement system, a part of the immune system.
Zimura is an aptamer, a chemically synthesized strand of RNA. Injected into the eye once a month, the drug is designed to bind to and block a complement system protein called C5. Iveric’s FDA submission is based on the results of two sham-controlled Phase 3 clinical trials that showed treatment with the drug slowed the rate of lesion growth measured at 12 months.
The first regulatory approval of a GA drug went to Apellis Pharmaceuticals’ Syfovre in February. That peptide drug is designed to block a different complement system protein called C3. Results from Phase 3 studies testing monthly and every-other-month injections of Syfovre showed reductions in the rate of lesion growth measured at 24 months. Neither the Apellis drug nor the Iveric drug have shown yet that slowing lesion growth preserves vision or slows the rate of vision loss. Assessing vision changes is one of the goals of an Apellis open label extension study that will continue to treat and follow patients for three years.
The size of the GA market leaves room for multiple treatment options, and Iveric’s upcoming FDA decision date puts it in a good position to become the second approved treatment for the condition. Astellas is already penciling in Zimura as a contributor to the company’s goal of reaching 1.7 trillion yen (about $12.3 billion) in sales by fiscal year 2025.

When Investment Rhymes with Canada
Canada has a proud history of achievement in the areas of science and technology, and the field of biomanufacturing and life sciences is no exception.
The prostate cancer drug Xtandi is currently Astellas’s biggest source of revenue, accounting for 534.3 billion yen (about $3.9 billion) in sales in the 2021 fiscal year, according to the company’s financial reports. The small molecule won its first FDA approval in 2012. More than half of Xtandi’s sales are from the U.S., where the drug is marketed by Pfizer under a collaboration agreement. The pact calls for the partners to share equally in profits from U.S. sales of the drug. Xtandi’s patents expire in 2027.
Astellas views Iveric as a key part of its growth strategy. The plan focuses on making investments in five areas: genetic regulation, immuno-oncology, blindness and regeneration, mitochondria, and targeted protein degradation. In the announcement of the acquisition, Astellas said Iveric provides a foundation of ophthalmology capabilities to drive the combined eye business going forward. In addition to GA, Iveric’s lead drug candidate is being evaluated in Stargardt disease, a rare inherited vision loss disorder with no FDA-approved therapies. The drug has reached Phase 2b testing in this indication.
“Iveric Bio has promising programs including avacincaptad pegol, an important program for geographic atrophy secondary to age-related macular degeneration, and capabilities across the entire value chain in the ophthalmology field,” Astellas President and CEO Naoki Okamura said in a prepared statement. “We believe that this acquisition will enable us to deliver greater value to patients with ocular diseases at high risk of blindness.”
Iveric’s pipeline includes gene therapies for vision-loss disorders, which also fits with Astellas’s gene therapy strategy. The Iveric gene therapies are in preclinical development as potential treatments for Leber congenital amaurosis type 10, Stargardt disease, and Usher syndrome type 2a. Earlier this year, Richard Wilson, Astellas’s senior vice president of genetic regulation, told MedCity News his company will focus its gene therapy efforts in neuromuscular, central nervous system, and ophthalmological indications. Astellas already has neuromuscular gene therapies lined up via a deal last year that gave it options to the Taysha Gene Therapies’ programs in giant axonal neuropathy (GAN) and Rett syndrome, though Taysha’s GAN program has encountered setbacks.
Astellas’s acquisition of Iveric has received approvals from the boards of directors of both companies, but still needs the approval of regulators as well as Iveric shareholders. The deal is expected to close in the third quarter of this year.
Photo by Flickr user Rakesh Rocky via a Creative Commons license












